Categroy List



Rubber Plungers for Prefilled Syringe

- 产品描述
-
Short Description
A pre-filled syringe is a disposable syringe that is already filled with the single dose of medication the patient needs to inject. By avoiding filling the syringe from a vial, the process is faster, easier and more accurate. It is commonly used for dental local anesthesia, vaccines, diabetes, autoimmune diseases and cancer.
Medicinal rubber plungers are widely used as primary packaging for various types of medicines. However, some relatively high-end packaging products such as prefilled syringes require rubber plunger have higher requirements for compatibility. First Rubber Company supplied products will make sure that our rubber components for prefilled syringes will meet all necessary functions.
We offer our customers integrity sealing solutions with the highest quality for their syringe systems. We are specialized to supply rubber plunger stoppers, rubber-lined aluminum seals, and needle shield for prefilled syringe.

The stopper (plunger or bung) is made of rubber that can be of different qualities and types. Today two classes of rubber are mainly used for prefilled syringes: one is chlorobutyl rubber, the other made of bromobutyl rubber.

The cap is located at the opposite end of the cartridge from the rubber plunger, and it is contained into an aluminum metal ring. The lined rubber is typically made of the same kind as the plunger rubber (usually bromobutyl rubber)
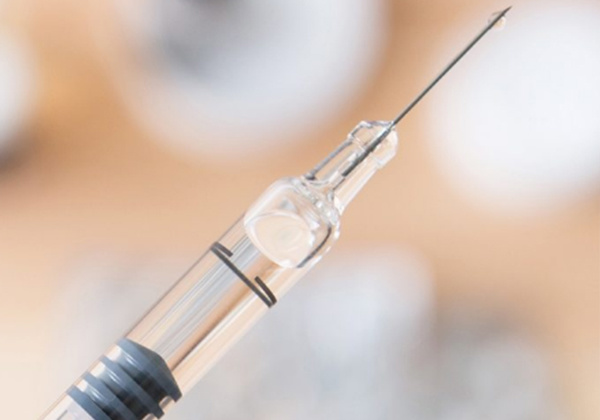
Protecting the needle from external forces is another important aspect when it comes to injectables. Both our soft and rigid needle shield that ensure the needle will not damaged during production, filling, and distribution.
Specifications
Item
Drawing No.
Feature
Diameter
Spiral Diameter
Spiral Height
Total Height
Rubber Plungers for Prefilled Syringe Use
Φ6.9 YGF
Pointed & 3 Lines
6.90±0.1
4
4.5
7.65±0.2
Φ9.15 YGF
Pointed & 4 Lines
9.15±0.15
——
——
7.7±0.4
10ML
Three Edges &
4 Lines10.00±0.10
——
——
9.50±0.2
15ML
Pointed & 3 Lines
12.62±0.1
7.4
7
16.06±0.2
20ML
Pointed & 3 Lines
19.41±0.1
10.9
7
14.97±0.2
50ML
Pointed & 3 Lines
29.59±0.1
17.5
7.85
16.56±0.2
Item
Specifications
Feature
Outer Diameter
Inner Diameter
Total Height
Needle Sheath for Prefilled Syringe Use
Softness
Rubber
7
4.7
23
Hard
Inner with rubber and outer with plastic
8.15±0.10
4.7
25
Advantage
1.High cleanliness standard. Production under 100,000 class cleaning area,
2.Superior service system, with professional technical personnel, to provide customers with supporting and personalized services at any time;
3.Supply well-known pharmaceutical manufacturers home and abroad.
Certificates

ISO 15378 Certification
Packing Details
Inner packing is PE bag; Outer packing is carton.
The most significant advantage of using a rubber plunger is its capacity to generate substantial force through a simple, user-friendly mechanism. When the rubber cup is positioned correctly over a drain opening and pressed downward, it displaces air; the subsequent pullback creates a powerful vacuum. This alternating push-pull action effectively dislodges obstructions, clearing pipes of materials that impede water flow. The robust, flexible nature of the rubber material is crucial here, as it forms a tight seal against various surface types—from porcelain and stainless steel to ceramic—ensuring that pressure is maintained and not lost during the plunging process. The efficacy of a high-quality rubber plunger in restoring proper drainage is often achieved within minutes, making it an exceptionally efficient tool.
Pre-filled syringe use rubber stoppers are essential components in pharmaceutical packaging, designed to ensure the safe storage, transportation, and administration of injectable medications. These stoppers create a secure, airtight, and contamination-resistant seal that protects drug stability throughout its shelf life. Manufactured from high-purity medical-grade elastomers, they are engineered to meet strict industry requirements for cleanliness, integrity, and compatibility with a wide range of formulations, including vaccines, biologics, peptides, and small-molecule injectable drugs.
A key function of these rubber stoppers is to prevent leakage and maintain sterility by forming a tight seal within the syringe barrel. Their elasticity allows repeated puncturing by needles or cannulas without compromising sealing performance, which is crucial for applications involving multiple withdrawals or compatibility testing. Stoppers for pre-filled syringes must also exhibit low levels of extractables and leachables to avoid chemical interactions that could affect drug efficacy or patient safety. To achieve this, manufacturers use advanced formulations, purification processes, and surface treatments that reduce particulate contamination, minimize ion release, and enhance biocompatibility.
Modern pre-filled syringe rubber stoppers are often coated with silicone oil or fluoropolymer materials to improve lubricity and reduce friction during plunger movement. This ensures smooth injection force, consistent dosing accuracy, and a comfortable injection experience for patients. Fluoropolymer-coated stoppers also offer enhanced barrier properties, preventing drug absorption and minimizing interactions between the formulation and the stopper surface. These coatings play a critical role in maintaining the long-term stability of sensitive biological drugs, which require carefully controlled packaging environments.
Dimensional precision is another vital characteristic. Rubber stoppers must fit securely within syringe barrels of varying sizes, such as 0.5 mL, 1 mL long, 1 mL short, 2.25 mL, and 5 mL formats. Precise dimensions ensure compatibility with automated filling and assembly equipment used in high-speed pharmaceutical production lines. Stoppers are designed to maintain consistent performance even under sterilization processes such as steam autoclaving, gamma irradiation, or ethylene oxide treatment. They retain elasticity, mechanical strength, and chemical stability after exposure to sterilization environments, ensuring safe and reliable use in clinical settings.
In addition to functional requirements, regulatory compliance is critical for pre-filled syringe rubber stoppers. High-quality stoppers adhere to global standards such as USP <381>, USP <382>, ISO 11040-4, and EP 3.2.9. Manufacturers perform rigorous testing, including permeability tests, compression force analysis, particulate evaluation, extractable studies, and cytotoxicity assessments. These tests guarantee that the stoppers meet the highest hygiene and performance standards required for pharmaceuticals intended for human injection.
Applications extend across a wide range of therapeutic areas. Rubber stoppers for pre-filled syringes are widely used for vaccines, insulin, anticoagulants, ophthalmic injections, monoclonal antibodies, emergency response medications, and other biologic or small-dose injectable therapies. Their role is increasingly important in self-injection devices and home-care treatments, where user-friendly syringes require stoppers that enable smooth operation, safe handling, and reliable dose delivery.
Overall, pre-filled syringe use rubber stoppers are indispensable components that contribute significantly to the reliability, safety, and convenience of modern injectable drug systems. Their precise engineering, high chemical purity, and excellent mechanical performance support the pharmaceutical industry’s growing demand for advanced, ready-to-use delivery solutions. By ensuring sterility, stability, and product integrity, these stoppers help maintain drug effectiveness and protect patient health throughout the entire lifecycle of the medication.
Get Quote Now
Feel free to leave us a message for any inquiry, we will respond within 24 hours.
Releate Products














